Q0245 Injection, bamlanivimab and etesevimab, 2100 mg...
Q0245 - Injection, bamlanivimab and etesevimab, 2100 mg
The above description is abbreviated. This code description may also have Includes, Excludes, Notes, Guidelines, Examples and other information.
Access to this feature is available in the following products:
- Find-A-Code Essentials
- Find-A-Code Professional
- Find-A-Code Premium
- Find-A-Code Elite
- Find-A-Code Facility Base
- Find-A-Code Facility Plus
- Find-A-Code Facility Complete
- HCC Standard
- HCC Pro
The above description is abbreviated. This code description may also have Includes, Excludes, Notes, Guidelines, Examples and other information.
Additional Code Information includes:
|
Save time with a Professional or Facility subscription! You will be able to see the most common modifiers billed to Medicare along with this code.

 HCPCS Index Entries (Reverse Index Lookup)
HCPCS Index Entries (Reverse Index Lookup)  reverse_index/reverse_index_content.php?set=HCPCS&c=Q0245
reverse_index/reverse_index_content.php?set=HCPCS&c=Q0245
View historical information about the code including when it was added, changed, deleted, etc. Access to this feature is available in the following products:
|
Subscribers may add their own notes as well as "Admin Notes" visible to all subscribers in their account. Access to this feature is available in the following products:
Subscribers may add their own notes as well as "Admin Notes" visible to all subscribers in their account. |
Subscribers will see related documentation, coding and billing tips. Access to this feature is available in the following products:
|

 DMEPOS Products (Durable Medical Equipment, Prosthetics, Orthotics, Supplies)
DMEPOS Products (Durable Medical Equipment, Prosthetics, Orthotics, Supplies)  dmepos/dmepos_content.php?set=HCPCS&c=Q0245
dmepos/dmepos_content.php?set=HCPCS&c=Q0245
Medicare COVID-19 Fees
Important: This product is not authorized for administration until further notice by the FDA.
| Labeler | Vaccine Procedure | Payment Allowance | Effective From | Effective To | Footnotes | My Fee |
|---|---|---|---|---|---|---|
| Eli Lilly | Injection, bamlanivimab and etesevimab, 2100 mg | $0.01 | 2021-02-09 | 2022-01-24 | [1][8][9] | (your fee) |
[1] Since we anticipate that providers, initially, will not incur a cost for the product, CMS will update the payment allowance at a later date. Providers should not bill for the product if they received it for free.
[8] On September 16, 2021, the FDA revised the EUA for bamlanivimab and etesevimab, administered together, to allow its use for post-exposure prophylaxis (PEP) in certain adult and pediatric patients. Providers and suppliers should use Q0245 and M0245 or M0246 to bill for administering bamlanivimab and etesevimab for PEP.
[9] On January 24, 2022, the FDA announced that, due to the high frequency of the Omicron variant, this product isn’t currently authorized in any U.S region and may not be administered for treatment or post-exposure prevention of COVID-19 under the EUA until further notice by the FDA.
Learn more at: https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/covid-19-vaccines-and-monoclonal-antibodies
This section shows APC information including: Status Indicator, Relative Weight, Payment Rate, Crosswalks, and more. Access to this feature is available in the following products:
|
View fees for this code from 4 different built-in fee schedules and from those you've added using the Compare-A-Fee™ tool. If you work with several fee schedules or would like to create custom fee comparison reports, you need our exclusive Compare-A-Fee™ tool.
|
View a table of UCR, Worker's Comp, and Medicare Fees here, as well as see UCR Fees in the charts below. Access to this feature is available in the following products:
Note: Subscribers will see the fee graphs below. |
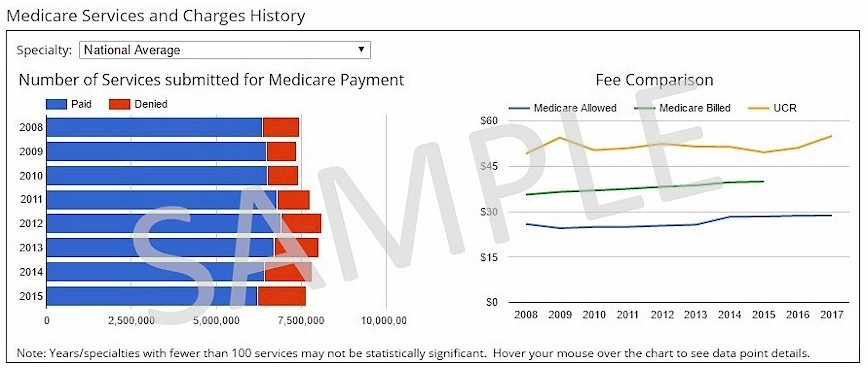
View a chart showing the last 8+ years of Medicare denial rates, Medicare Allowed amounts, and Medicare billed amounts. Access to this feature is available in the following products:
Note: Subscribers will see the fee graphs below. |
View relationships (or crosswalks) between code sets. Access to this feature is available in the following products:
|

 Medicare Policies & Guidelines (Articles, LCDs, NCDs)
Medicare Policies & Guidelines (Articles, LCDs, NCDs)  coverage/coverage_content.php?set=HCPCS&c=Q0245
coverage/coverage_content.php?set=HCPCS&c=Q0245
Thank you for choosing Find-A-Code, please Sign In to remove ads.






 Quick, Current, Complete - www.findacode.com
Quick, Current, Complete - www.findacode.com