M0241 Intravenous infusion or subcutaneous injection, casirivimab and imdevimab includes infusion or injection, and post...
M0241 - Casiri and imdev repeat hm
Need more information about M0241? Get access to fees, crosswalks, billing policies, similar codes and much more.
Access to this feature is available in the following products:
- Find-A-Code Essentials
- Find-A-Code Professional/Premium/Elite
- Find-A-Code Facility Base/Plus/Complete
- HCC Standard/Pro
Sign in or talk to our friendly, US-based customer support team to determine exactly which subscription is right for you and your team.
Additional Code Information includes:
Access to this feature is available in the following products:
|
Save time with a Professional or Facility subscription! You will be able to see the most common modifiers billed to Medicare along with this code.

 HCPCS Index Entries (Reverse Index Lookup)
HCPCS Index Entries (Reverse Index Lookup)  reverse_index/reverse_index_content.php?set=HCPCS&c=M0241
reverse_index/reverse_index_content.php?set=HCPCS&c=M0241
View historical information about the code including when it was added, changed, deleted, etc. Access to this feature is available in the following products:
|
Subscribers may add their own notes as well as "Admin Notes" visible to all subscribers in their account. Access to this feature is available in the following products:
Subscribers may add their own notes as well as "Admin Notes" visible to all subscribers in their account. |
Subscribers will see related documentation, coding and billing tips. Access to this feature is available in the following products:
|

 DMEPOS Products (Durable Medical Equipment, Prosthetics, Orthotics, Supplies)
DMEPOS Products (Durable Medical Equipment, Prosthetics, Orthotics, Supplies)  dmepos/dmepos_content.php?set=HCPCS&c=M0241
dmepos/dmepos_content.php?set=HCPCS&c=M0241
* Note: Medicare may or may NOT reimburse you for this code. The fees provided below are based on values established by CMS/Medicare. Please check with your local Medicare contact on whether this code is eligible for reimbursement.
Medicare vs. My Fee Evaluation
| Modifier | Medicare Allowed | 150% | 200% | My Fee |
|---|---|---|---|---|
| (none) | $0.00 | $0.00 | $0.00 | (your fee) |
Access to calculated fee values is available. Access to this feature is available in the following products:
|
Medicare Participating - Assignment Accepted (Mandatory)
| Modifier | Allowed | Medicare 80% | Patient Pays |
|---|---|---|---|
| (none) | $0.00 | $##.## | $##.## |
Medicare Non-Participating - Assignment Accepted (Check To Doctor)
| Modifier | Allowed | Medicare 80% | Patient Pays | Limiting Charge (Amount Billed) |
|---|---|---|---|---|
| (none) | $##.## | $##.## | $##.## | $##.## |
Medicare Non-Participating - Assignment NOT Accepted (Check To Patient)
| Modifier | Allowed | Medicare 80% | Patient Pays | Limiting Charge (Amount Billed) |
|---|---|---|---|---|
| (none) | $##.## | $##.## | $##.## | $##.## |
Medicare vs. My Fee Evaluation
| Modifier | Medicare Allowed | 150% | 200% | My Fee |
|---|---|---|---|---|
| (none) | $0.00 | $0.00 | $0.00 | (your fee) |
Access to calculated fee values is available. Access to this feature is available in the following products:
Note: Subscribers will see the calculated values below. |
Medicare Participating - Assignment Accepted (Mandatory)
| Modifier | Allowed | Medicare 80% | Patient Pays |
|---|---|---|---|
| (none) | $0.00 | $##.## | $##.## |
Medicare Non-Participating - Assignment Accepted (Check To Doctor)
| Modifier | Allowed | Medicare 80% | Patient Pays | Limiting Charge (Amount Billed) |
|---|---|---|---|---|
| (none) | $##.## | $##.## | $##.## | $##.## |
Medicare Non-Participating - Assignment NOT Accepted (Check To Patient)
| Modifier | Allowed | Medicare 80% | Patient Pays | Limiting Charge (Amount Billed) |
|---|---|---|---|---|
| (none) | $##.## | $##.## | $##.## | $##.## |
This section shows APC information including: Status Indicator, Relative Weight, Payment Rate, Crosswalks, and more. Access to this feature is available in the following products:
|
View fees for this code from 4 different built-in fee schedules and from those you've added using the Compare-A-Fee™ tool. If you work with several fee schedules or would like to create custom fee comparison reports, you need our exclusive Compare-A-Fee™ tool.
Access to this feature is available in the following products:
|
View a table of UCR, Worker's Comp, and Medicare Fees here, as well as see UCR Fees in the charts below. Access to this feature is available in the following products:
Note: Subscribers will see the fee graphs below. |
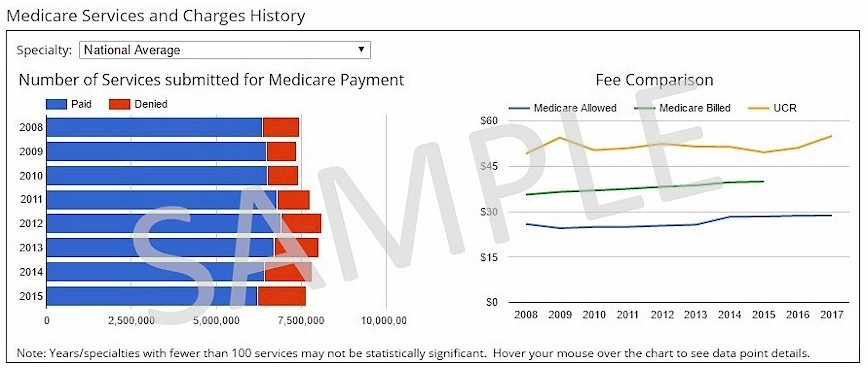
View a chart showing the last 8+ years of Medicare denial rates, Medicare Allowed amounts, and Medicare billed amounts. Access to this feature is available in the following products:
Note: Subscribers will see the fee graphs below. |
* Note: Medicare may or may NOT reimburse you for this code. The fees provided below are based on values established by CMS/Medicare. Please check with your local Medicare contact on whether this code is eligible for reimbursement.
| Modifier | Work | Practice Expense | Malpractice Expense | Total |
|---|---|---|---|---|
| (none) | 0.000 |
Access to calculated fee values is available. Access to this feature is available in the following products:
|
| Pre-Service | Intra-Service | Post-Service | Total Time* |
|---|---|---|---|
| ## | ## | ## | ## min |
| Modifier | National Unadjusted Work RVU | Work GPCI | Adjusted Work RVU |
|---|---|---|---|
| (none) | ##.## | ##.## |
| Staff | Staff Rate | Pre Time | Intra Time | Post Time | Total Time |
|---|---|---|---|---|---|
| $0.00 / min | ## min | ## min | ## min | ## min |
| Item | Purchase Price | Expected Life | Total Time |
|---|---|---|---|
| $##.## | ## years | ## min |
| Item | Unit Price | Quantity | Unit | Amount |
|---|
| Modifier | National Unadjusted PE RVU | PE GPCI | Adjusted PE RVU |
|---|---|---|---|
| (none) | ##.## | ##.## | 0.000 |
| Modifier | Work | Practice Expense | Malpractice Expense | Total |
|---|---|---|---|---|
| (none) | 0.000 |
Access to calculated fee values is available. Access to this feature is available in the following products:
Note: Subscribers will see the calculated values below. |
| Pre-Service | Intra-Service | Post-Service | Total Time* |
|---|---|---|---|
| ## | ## | ## | ## min |
| Modifier | National Unadjusted Work RVU | Work GPCI | Adjusted Work RVU |
|---|---|---|---|
| (none) | ##.## | ##.## |
| Staff | Staff Rate | Pre Time | Intra Time | Post Time | Total Time |
|---|---|---|---|---|---|
| $0.00 / min | ## min | ## min | ## min | ## min |
| Item | Purchase Price | Expected Life | Total Time |
|---|---|---|---|
| $##.## | ## years | ## min |
| Item | Unit Price | Quantity | Unit | Amount |
|---|
| Modifier | National Unadjusted PE RVU | PE GPCI | Adjusted PE RVU |
|---|---|---|---|
| (none) | ##.## | ##.## | 0.000 |

 Cross-A-Code™ (ICD-9/10, CPT, Modifiers, NCCI, NDC, ASA CROSSWALK®)
Cross-A-Code™ (ICD-9/10, CPT, Modifiers, NCCI, NDC, ASA CROSSWALK®)  crosswalks/crosswalk_content.php?set=HCPCS&c=M0241
crosswalks/crosswalk_content.php?set=HCPCS&c=M0241
View relationships (or crosswalks) between code sets. Access to this feature is available in the following products:
|

 Medicare Policies & Guidelines (Articles, LCDs, NCDs)
Medicare Policies & Guidelines (Articles, LCDs, NCDs)  coverage/coverage_content.php?set=HCPCS&c=M0241
coverage/coverage_content.php?set=HCPCS&c=M0241
Thank you for choosing Find-A-Code, please Sign In to remove ads.





 Quick, Current, Complete - www.findacode.com
Quick, Current, Complete - www.findacode.com